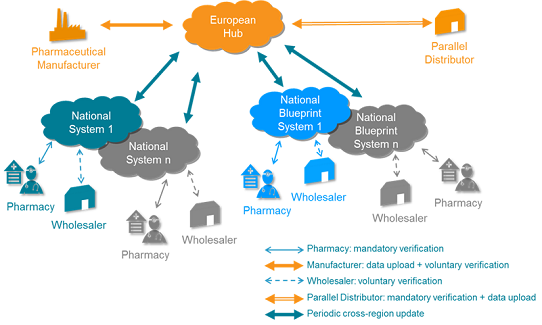
The Verification system consists of an European hub, the EMVS system to which the product information is uploaded and the DMVS, a national system, where the verification of the packs are performed.
The European hub is administered by the European Medicines Verification Organisation (EMVO) which contains data of all packs being sold in EU. This information comes from manufacturers and parallel importers (Marketing Authorisation Holders - MAHs) uploading information into the hub. This is a condition for being able to sell their products. National organisations have been set up in every country to operate their national database. In Denmark, this is assigned to the DMVO. The national database receives information from wholesalers and pharmacies on which products are distributed in each country and hereafter this information is transferred to the European hub.
>>Uploading product data to the European hub is a pre-condition for selling prescribed products from February 9, 2019<<
The European hub sends data to the national database in order to verify that the product has not been sold elsewhere in EU. The national database forwards this data to wholesalers /pharmacies. It can only be dispensed when it has been verified that the product has not been sold elsewhere.
The organisation underlying the verification system is as follows: